|
Imagine a world where doctors and scientists could understand the most fundamental causes of human blindness. For Rick Cote, professor in the Department of Molecular, Cellular and Biomedical Sciences (MCBS), it is this ultimate goal — coupled with an innate curiosity about how vision works at the molecular level — that fuels his passion for research.
First drawn to the fascinating nature of enzymes while a student, Cote decided to focus his career in vision research because of the field’s potential benefits for patients coping with loss of vision. “I am fascinated by how enzymes work, and the highly regulated enzyme that controls vision — PDE6 — is amazingly good at being an enzyme,” Cote explains.
With more than two decades of funding from the National Eye Institute of the National Institutes of Health, Cote’s research team has focused on the retina, studying the photoreceptors (rods and cones) that transform light into nerve impulses. The phosphodiesterase enzyme in photoreceptors (called PDE6) is necessary to trigger the nerve signal that is the first step in vision. However, genetic mutations in PDE6 or other photoreceptor proteins can disrupt this process, such as in retinitis pigmentosa, a progressive, inherited disorder that affects 100,000 people in the U.S. and 1.5 million people worldwide.
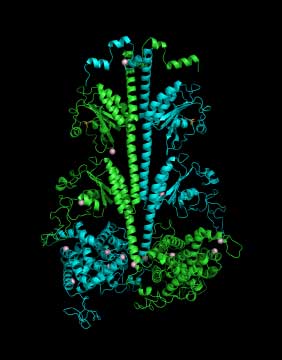 |
| Three-dimensional model of the PDE6 enzyme responsible for the first steps in vision |
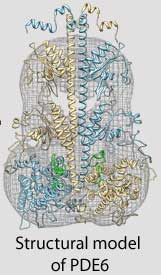
Cote and his colleagues use structural biology, proteomics, biochemical approaches and sophisticated imaging techniques to study the three-dimensional structure and complex regulation of PDE6 during the very first steps in the visual signaling pathway. For example, because the atomic-level structure of many proteins in the retina have not yet been determined, Feixia Chu, associate professor in MCBS, uses her expertise in proteomics to assist Cote in cross-linking PDE6 with other proteins whose structures have been solved. This new technique could lead to important insights into how the proteins that control the vision process function normally, and what goes wrong in the retinas of people with retinitis pigmentosa or other visual disorders.
It is this type of interdisciplinary, teamwork-driven research collaboration that continues to motivate Cote. The team, currently composed of a senior scientist, a lab manager, three biochemistry doctoral students and three undergraduate students, is key to the lab’s productivity and Cote’s professional satisfaction. Cote notes, “While I consider myself an introvert, what gives me the most delight is working with colleagues, staff and students in the process of carrying out research.”
 |
Cote compares his ongoing work to peeling an onion. “There are always new layers to peel back and reveal, and my research continues to take me down new paths of inquiry,” he says. While his efforts and contributions to his field have been highly regarded and personally satisfying, Cote also points out a major challenge of a career in scientific research: “Research is a process of delayed gratification; each breakthrough in knowledge is preceded by many failed experiments, misconceived hypotheses and progressive refinement of an experimental design that eventually leads to unambiguous outcomes. For me, this is the essence of creativity in the lab.”
Cote’s research on the enzyme PDE6 focuses on a part of our natural world that is physically quite small yet remarkably complex, but the potential impact of his vision research program is more tangible. Years of research on the human eye by Cote and many others have led to new discoveries that bring us closer to a full understanding of the molecular events that occur during the first steps in vision. For Cote and his colleagues, a future in which diseases of the retina are understood and effectively treated is not a dream, but a tangible goal that soon could be reached.
 |
The enzyme PDE6 is one of a group of related enzymes called Class I cyclic nucleotide phosphodiesterases (PDEs). While present in all vertebrates, there are no known Class I PDEs present in plants. Professor Rick Cote believes that this fact makes PDEs attractive targets for developing compounds that inhibit PDEs present in animal pests that reduce yields of agriculturally important crops.
Plant parasitic nematodes, such as root-knot nematodes (shown at right), have six of the 11 PDE families found in vertebrates, namely PDE1, PDE2, PDE3, PDE4, PDE8 and PDE10. Based on differences in the amino acid sequence of nematode PDEs and their vertebrate counterparts, Cote and his colleagues hypothesize that compounds targeting PDEs in plant parasitic nematodes can be developed that lack adverse effects on vertebrates or on plants.
With funding from the NH Agricultural Experiment Station, Cote’s research team recently has found that PDE inhibitors developed by pharmaceutical companies to combat a variety of human health conditions also interact with nematode PDE enzymes. Their research also has shown that certain PDE inhibitors substantially reduce nematode motility, and may impair the ability of plant parasitic nematodes to infect plant roots. Disrupting the nematode life cycle with PDE inhibitors may represent a novel, non-toxic approach for chemical control of plant parasitic nematodes that afflict agricultural crops.
-
Written By:
Paige Belisle | Freelance Writer -
Written By:
Michael Thompson | Freelance Writer -
Written By:
Lynnette Hentges | Freelance Writer