The Presence of 17-beta Estradiol, 17-alpha Ethinyl Estradiol, and Estrone in Wastewater Treatment: Navigating Unanticipated Results
As of 2020, 14 percent of women in America ages 15 to 49 were using birth control pills (CDC 2021). Oral contraceptives have two main components: estrogen and progesterone. Studies have shown that when released into aquatic environments, estrogenic compounds can lead to the feminization of male fish. Estrogen has been shown to stimulate the production of vitellogenin, an egg yolk precursor, in male fish, inducing the partial development of ova in the testes (Nazari and Suja 2016). Reduced testes size, lower sperm count, and decreased reproductive fitness also result from environmental exposure to estrogen. Ultimately, these fish are not able to reproduce properly, leading to a decrease in fish populations and disruption of the aquatic food chain (Adeel et al.). In addition, humans who consume fish exposed to these hormones are at a greater risk of cancer and cardiovascular disease (Adeel et al. 2016).

The author, Alexis Eaton
As someone who has taken medication, I’ve always assumed that my body absorbs all of the compounds in the pharmaceuticals. I never considered that excess chemicals might be discharged from the body, move through wastewater treatment plants, and have an impact on aquatic environments. Similarly, most people who take birth control don’t think about the fate of oral contraceptives after they take “the pill.” As an environmental engineering and sustainability dual major with a passion for environmental stewardship I became interested in the fate and transport of pharmaceuticals and other emerging contaminants and was eager to participate in the Research Experience and Apprenticeship Program (REAP) at the University of New Hampshire (UNH) so I could explore this topic further.
Through REAP and with Dr. Paula Mouser and several graduate students in the UNH Department of Environmental Engineering, I studied how two types of estrogen, 17-beta estradiol and 17-alpha ethinyl estradiol, move through treatment processes at wastewater facilities. My goal was to understand how effective each treatment process is at targeting said compounds. By working toward this goal, I gained a deeper understanding of the many factors at play in the fate of emerging contaminants (pollutants that may pose a risk to environmental or human health but are not yet regulated under environmental laws) and the factors at play in wastewater treatment as a whole. My project yielded surprising results, so to draw meaningful conclusions I had to further examine the (bio)chemistry of estrogenic compounds and the limitations of the tools used to measure emerging contaminants.
Wastewater Treatment Principles
Discharge from wastewater treatment facilities is the main source of estrogen in aquatic environments; therefore, it is vital that these compounds be removed during the treatment process. The overall goal of wastewater treatment is to reduce the concentrations of certain constituents that have been deemed harmful to the environment or to public health. There are three stages of treatment, which use various treatment processes: primary treatment, which focuses on solid removal; secondary treatment, which reduces the demand for oxygen; and disinfection. Wastewater treatment yields two products: the treated effluent, which is commonly discharged to a nearby waterway, and sludge, which is defined as the solid portion of wastewater. In activated sludge treatment, wastewater is aerated and then passed to a settling tank, where the sludge settles to the bottom, leaving on top the liquid portion, which has been mechanically and biologically treated. The sludge is processed separately and is often recycled as a soil amendment. A diagram of the wastewater treatment process is shown in Figure 1.

Figure 1: Wastewater Treatment Plant Flow Diagram, Wallingford, Connecticut. The top portion of the figure depicts the wastewater treatment portion of the facility. The bottom portion depicts how the activated sludge is stored and treated. The activated sludge is introduced into the wastewater through the reaeration channel, which is shown in both portions of the figure. Sample locations are indicated by number (see key).
I aimed to better understand the presence and concentration of estrogenic compounds in wastewater facilities and to evaluate which treatment process best removes hormones. The project opened my eyes to the effects of pharmaceuticals on aquatic species, and it demonstrated the important role that wastewater treatment plants have in mitigating environmental harm. Ultimately, my research underscored the complex nature of estrogenic compounds and the need for proper removal of hormones from wastewater.

The Quinnipiac River in Wallingford, Connecticut.
Location of Research and Data Collection
This study was conducted in collaboration with the wastewater treatment facility in Wallingford, Connecticut, the town where I grew up. I decided to make the three-hour trip to my hometown’s treatment plant rather than sample from a New Hampshire treatment plant because of the connection I have to the town and the local environment. The Wallingford facility discharges effluent into the Quinnipiac River. I have fond memories of walking alongside the river as a child and throwing rocks in whenever I had the opportunity. I remember taking my first dog, Max, for a stroll on the Quinnipiac River Linear Trail and having to give him coffee from a Dunkin’ Donuts cup to help him hydrate on that hot summer day because the river water wasn’t safe to drink. By examining hormone concentrations in a facility that discharges effluent into the Quinnipiac River, I knew that my research would give me a better understanding of how the river that has been a part of my childhood could be negatively impacted in the near future. Comprehensively, the project allowed me to analyze the overall effectiveness of the facility and to help ensure that the health of the Wallingford community is being preserved.
Sampling and Analysis
My project was divided into two phases. I spent the first half of my summer on campus and at the treatment plant and the second half at home, where I analyzed my findings. While on campus, I was trained in conducting lab work by my faculty adviser, Dr. Mouser, and several of her graduate students. After my training was complete, a graduate student drove me to Wallingford so I could sample at the treatment plant.
I obtained six samples during my trip to Wallingford, five of which were wastewater taken from various stages in the treatment process and one of which was digested, or anaerobically decomposed, sludge, taken from the secondary digester. Each sample was collected from a different location in the facility. An influent sample (untreated wastewater) was taken, as well as samples from the anaerobic selector, the rotating biological contactors, the secondary settling tanks, and the UV disinfection tanks. (See Figure 1.)

The author holds a sample collected at the Wallingford wastewater treatment plant with the help of graduate student Carmela Antonellis.
I used the automatic samplers that are available throughout the plant to collect samples at each location. An automatic sampler consists of a plastic tube inside a sealed box; when the handle inside the box is rotated, wastewater can flow out of the tube and into collection vials. However, for the secondary settling tanks, I had to use a surface water sampling method, because an automatic sampler wasn’t available. During sampling, safety precautions were taken: I wore a hard hat, gloves, a lab coat, and a mask. At each sampling location I filled one-liter bottles with wastewater; the water within these bottles was used to perform each of the contextual tests listed in Figure 2. I also filled smaller amber bottles with wastewater at each location; these samples were sent to Weck Laboratories in California for analysis of a predetermined suite of hormones: 17-beta estradiol, 17-alpha ethinyl estradiol, progesterone, testosterone, and estrone. All samples were stored in a cooler and placed in a refrigerator immediately upon arrival at the University of New Hampshire. The samples for the contract lab were shipped out the following day.
Obtaining a sample from the river that the plant effluent is released into was part of my initial research plan. The purpose of such a sample was to determine whether the estrogenic compounds being studied were present in substantial amounts in the Quinnipiac River. Unfortunately, there was not an access point downstream of the treatment plant safe enough to sample at. Having this information would have been useful for determining whether fish species in the river could be negatively impacted in the near future.
I used a variety of lab equipment to conduct the contextual tests, which provided me with background information about the samples. Figure 2 describes the contextual tests performed. These parameters are always measured by treatment facilities to assess the plant’s performance and monitor contaminant levels. I decided to conduct such tests myself to gain more experience in the lab as well as to get a better understanding of the plant’s performance while I waited for the results from Weck Lab.
Once I had completed the contextual tests at UNH, I went home and analyzed my results. This involved performing calculations, graphing my data with Microsoft Excel, and using online research publications to frame the results of the study.
Contextual Test Results
From my work in Dr. Mouser’s lab, I was able to measure wastewater characteristics as well as compare my measurements with Connecticut’s wastewater standards. I learned that the pH stayed relatively constant and within the allowed range as the treatment process progressed. The amount of dissolved oxygen in the wastewater increased significantly from start to finish, meaning it became more suitable for aquatic species. The conductivity of the wastewater decreased significantly as the treatment process progressed; this decline is characteristic of the treatment process and indicates that pollutants are being removed through treatment.

Figure 2: Parameters measured and equipment used to conduct the contextual tests.
Chemical oxygen demand is a measure of the amount of oxygen needed to oxidize organic matter. The results of the tests showed that the chemical oxygen demand decreased significantly during treatment, which is a sign of organic matter being broken down and of increasing water quality, indicating that the facility is performing well.
Orthophosphate as phosphorus decreased significantly from start to finish in the treatment process. It is vital that treatment plants remove excess phosphorus from wastewater to prevent eutrophication, the process by which a body of water becomes too rich in nutrients. In sample location four, the secondary settling tanks, where samples are taken at the surface, no orthophosphate was found. This can be attributed to the fact that the sludge settles out to the bottom of these tanks, taking the phosphorus with it. This demonstrates the effectiveness of activated sludge treatment; when sludge is separated from the liquid portion of the wastewater, contaminants are removed.
The amount of nitrogen ammonia present in the samples decreased as the treatment process progressed, which also demonstrates how effective the treatment process is. Ammonia is a toxic pollutant that leads to algal and plant growth in water bodies. The concentration of the compound in the UV disinfection tank sample was below 4 mg/L. UV disinfection is the last stage in the treatment process, meaning the sample’s concentration of nitrogen ammonia did not exceed the average limit for the month of June (CT DEEP 2013).
The solids content in the wastewater decreased as the treatment process progressed, which is to be expected. It is vital that treatment plants remove the solid components of the wastewater from the liquids so that only liquid effluent is discharged into water bodies. For the total suspended solids, total volatile solids, total dissolved solids, and total fixed solids analyses, I filtered the solids off from the liquid portion in the samples and then put the solids in a 105℃ laboratory oven for four hours. I calculated the changes in mass before and after heating, and I used the appropriate equations to calculate the solid concentrations.
Hormone Results and Challenges
After I ran all the contextual tests, I received results from Weck Laboratories about the hormone concentrations, and those results completely changed the direction of my research. Surprisingly, the contract lab did not detect 17-beta estradiol or 17-alpha ethinyl estradiol, which are commonly found in birth control pills, in any of the samples. Ultimately, 17-beta estradiol and 17-alpha ethinyl estradiol may have been present in the samples, but not in amounts detectable using the analytical approach that Weck Lab used.
I was devastated by the results. I reached out to Dr. Mouser right away, and she recommended looking at the metabolic pathway of estrogen to learn more about the one compound that was detected in the samples: estrone. Estrone is an estrogenic compound naturally released by the female body, especially after menopause or during pregnancy.
After reviewing the metabolic pathway, I realized that under certain aerobic conditions and in the presence of certain microorganisms, ethinyl estradiol can be converted to estrone. Furthermore, estradiol can also be converted to estrone in the presence of particular microorganisms and certain environmental conditions (Adeel et al. 2016). Reviewing the (bio)chemical and microbial metabolic pathway of estrogen (Figure 3) as well as the data obtained from the contract lab led me to believe that the conditions in the treatment facility allowed for the conversion of 17-beta estradiol and 17-alpha ethinyl estradiol to an amount of estrone large enough to be detected in the wastewater facility and to pose a threat to aquatic species. It is important to note that studies have shown that even concentrations ranging from 1 to 2 ng/L of estrogen have negative impacts on fish populations (Nazari and Suja 2016), making the detection of estrone relevant to aquatic health.
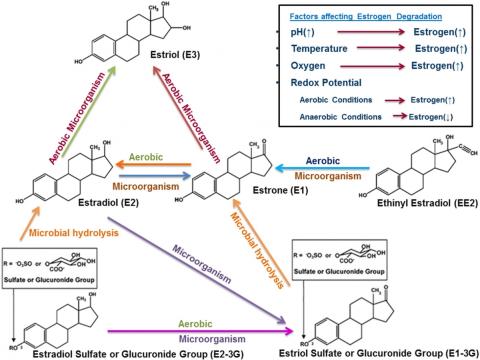
Figure 3: Metabolic Pathway of Estrogen (Source: Adeel et al. 2016)
Although I was unable to directly address my original research question about the presence of estradiol and ethinyl estradiol, I was able to draw interesting conclusions about the properties of estrone, where it might have come from, and how the compound moves through the facility.
The total suspended solids data that I gathered in Dr. Mouser’s lab reveals that locations one and three (see Figure 1) had the most suspended solids. According to the estrone results, no estrone was detected in locations one or three. I learned during my research that estrone has a Koc range of 457–18,000, meaning the compound adsorbs, or “adheres,” to solids (NCBI n.d.). When a compound prefers to interact with solids over liquids, measurement results in the liquids can be obscured.
During processing at Weck Lab, the samples were leached, underwent a solid phase extraction, and were then eluted. In other words, soluble matter was removed from the solids with a liquid solvent, and the compounds that had dissolved in the solvent were separated based on their physical and chemical properties. The data suggests that the adsorbed estrone might not have been properly eluted (removed with a solvent) and that some might have remained on the extracted solids, going unmeasured in the sample. This may have been the case even though no estrone was detected in the solid sample.
Upon reviewing the detection limits of the solid samples versus the wastewater samples, it is apparent that the estrone in the solid sample needed to be present in a much higher amount than the estrone in the wastewater sample in order to surpass the detection limit. Therefore, estrone may have been present in the solid sample; however, there wasn’t enough of it to surpass the detection limit of 15 parts per billion during analysis. To determine this, I had to perform calculations and call Weck Laboratories to obtain more information on how the samples were processed.
Ultimately the results from the contract lab did not allow me to determine which wastewater treatment method used at the Wallingford facility best targets the removal of 17-beta estradiol and 17-alpha ethinyl estradiol. However, the results do prove that estrone exists in significant quantities in the wastewater at the Wallingford facility, and fish species in the Quinnipiac River (the river into which the treatment plant discharges effluent) could be impacted if it is not possible to mitigate the compound’s presence. This study also provides some evidence that under certain biological and environmental conditions, estradiol and ethinyl estradiol may be converted to estrone during wastewater treatment or in the human gut before it reaches the wastewater treatment facility.
Conclusion
In the future, I would like to repeat this study during the academic year and sample from the wastewater treatment facility serving UNH in Durham, New Hampshire. It is likely that the percentage of the population that uses oral contraceptives in a college town is higher than the percentage in a town like Wallingford, Connecticut, that is not home to a large university. Consequently, estrogenic compounds would probably be present in much larger concentrations, which, perhaps, would be large enough to surpass the detection limits; thus, the equipment used to measure hormone levels may be able to detect a wider range of estrogenic compounds. This would allow me to get a better understanding of the fate and transport of hormones in wastewater.
I’ve learned that research isn’t perfect, and I won’t always get the results I’m hoping for; I would advise other undergraduate researchers not to get discouraged by this. I was heartbroken that the contract lab didn’t detect the compounds I was looking for, but after doing more research, I was still able to draw meaningful conclusions from the data. My work highlights the challenges in detecting and tracking emerging contaminants in complex samples such as wastewater, where they may interact with suspended solids. It takes consistency and persistence to contribute to the growing body of knowledge surrounding emerging contaminants and related fields. I look forward to continuing my studies at UNH, and I plan to continue my pursuit of environmental stewardship through participation in research opportunities.
I am incredibly grateful for this research opportunity, and I hope to take advantage of a different fellowship offered by the Hamel Center for Undergraduate Research after my sophomore year. Special thanks to Dr. Mouser and her research team, specifically, Carmela Antonellis, Sydney Adams, Cassidy Yates, Mina Aghababaei Shahrestani, and Kellen Sawyer. Part of the analysis for this research project was funded through a New Hampshire Sea Grant award to Dr. Mouser. This project couldn’t have been completed without the help of Kim Maloney, environmental laboratory director at the Wallingford, Connecticut, Wastewater Treatment Facility. Special thanks also goes to Dr. Laura Thompson and Mr. Dana Hamel for their generous donations to the Hamel Center for Undergraduate Research; this project could not have been funded without their support.
References
Adeel, Muhammad, et al. “Environmental Impact of Estrogens on Human, Animal and Plant Life: A Critical Review.” Environment International, Pergamon, 29 Dec. 2016, www.sciencedirect.com/science/article/pii/S0160412016304494.
CDC (Centers for Disease Control and Prevention). “Contraceptive Use in the United States.” 10 Nov. 2020, www.cdc.gov/nchs/fastats/contraceptive.htm.
CT DEEP (Connecticut Department of Energy and Environmental Protection).Town of Wallingford WPCF, CT0100617, Draft Permit. 18 Oct. 2013, https://www.epa.gov/sites/default/files/2019-07/documents/draftct0100617permit.pdf.
Nazari, Emad, and Fatihah Suja. “Effects of 17β-Estradiol (E2) on Aqueous Organisms and Its Treatment Problem: A Review.” De Gruyter, 24 Nov. 2016, www.degruyter.com/document/doi/10.1515/reveh-2016-0040/html.
NCBI (National Center for Biotechnology Information). “Estrone.” PubChem Compound Database, U.S. National Library of Medicine, n.d., pubchem.ncbi.nlm.nih.gov/compound/5870#section=Ecological-Information.
Author and Mentor Bios
Alexis Eaton, a sophomore from Wallingford, Connecticut, is an environmental engineering and sustainability dual major at the University of New Hampshire (UNH). Alexis is a member of the University Honors Program and the Tau Beta Pi engineering honor society. She conducted her research on hormone concentrations in wastewater through the Research Experience and Apprenticeship Program (REAP). She feels that it was a rewarding process and that she has grown by taking part in this opportunity. Alexis found that the most satisfying part of research was getting results that made sense; she saw trends in preliminary tests that she could put into context and explain. Because of the pandemic, she had to be cautious about working with wastewater samples, because COVID-19 could be present in them. This reality allowed her to practice lab safety skills, which she hopes to further develop this summer by partaking in a Summer Undergraduate Research Fellowship (SURF) to continue studying emerging contaminants. Alexis hopes her Inquiry article will raise awareness about her research topic as well as inspire undergraduates to take advantage of research opportunities. Following graduation in 2024, Alexis hopes to work for the Environmental Protection Agency or the National Park Service and anticipates that grad school will be a part of her future. Writing for Inquiry allowed her to practice skills that she hopes to use in her professional career.
Dr. Paula Mouser, P.E., is an associate professor in the Department of Civil and Environmental Engineering. She joined the University of New Hampshire (UNH) in August 2017; her teaching specializations are environmental engineering microbiology and environmental pollution, and her research focuses on microbial transformation of environmental pollutants. She conducts research related to emerging contaminants, such as PFAS and PPCPs, that is funded by the NH Sea Grant. Alexis’s project dovetailed with research on this broader topic. Dr. Mouser enjoyed mentoring Alexis, who is one of the most motivated, independent undergraduate researchers she has mentored. She felt that Alexis took advantage of opportunities to work in the lab and the field and to learn from graduate students. Alexis is Dr. Mouser’s first Inquiry author, and she feels that the process of writing for Inquiry is similar to writing a paper for a peer-reviewed journal: it requires substantial time to develop, multiple iterations of writing and editing, and patience to polish the paragraphs in a way that improves readability. In her words, “It’s an awesome experience for a student to be mentored through this process and to have an opportunity to see their work in print for a wide audience to read.”
Copyright 2022, Alexis Eaton
