Total Sediment Mercury Above and Below the Mill Pond Dam in Durham, New Hampshire
Mercury is a pervasive environmental contaminant, and it has multiple pathways into ecosystems and watersheds. The primary pathway of mercury distribution is through the atmosphere, where gaseous mercury remains aloft for up to a year and can be transported long distances. A secondary pathway of mercury distribution is through point sources, which are identifiable sources of pollution or contaminants. Once mercury is introduced into landscapes, it has the potential to migrate into other environments, where it can enter marine and freshwater aquatic ecosystems.
It is important to study mercury because specific types of mercury are indicators of environmental health. This health is not directly linked to atmospheric mercury concentrations or even to total amounts of mercury, but production of the bioaccumulative methylmercury is a chief source of environmental concern (Driscoll et al., 2013). Methylmercury targets the central nervous system, and its toxicity as a neurotoxin has been known since the 1950s (Castoldi et al., 2003). Studies show that mercury can transform into methylmercury under certain environmental conditions, so studying mercury contents in the environment helps to identify potential places of concern.

Mill Pond Dam in Durham, NH.
The Mill Pond Dam, a head-of-tide dam in Durham, New Hampshire, separates tidal waters from fresh water in the Oyster River. Though the Town of Durham voted to remove the dam in March 2022, the dam is still in place as of March 2024 and remains scheduled for removal in the forthcoming years (Dandurant, 2022; New Hampshire Department of Environmental Services, n.d.). Below the Mill Pond Dam lies the Great Bay Estuary environment, which is an area where fresh water from the Oyster River mixes with salt water that drains out to the Atlantic Ocean. Freshwater environments above the dam include the Mill Pond, College Brook, and the Oyster River.
Prior research has identified areas with elevated levels of total mercury in cored sediments in Mill Pond (Miller, 2020). This project seeks to investigate these findings in greater detail by evaluating how mercury concentrations, together with organic matter, vary above and below the Mill Pond Dam. This project was conducted in 2023 with a Summer Undergraduate Research Fellowship (SURF) from the Hamel Center for Undergraduate Research at the University of New Hampshire.
Project Overview and Methods
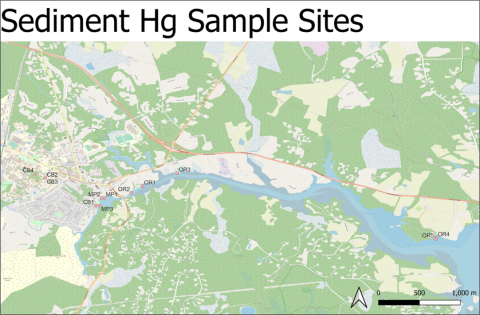
Figure 1. Map of sediment mercury sample sites. Sample sites in the Oyster River: Old Landing (sites OR1 and OR2); Jackson’s Landing (OR3); Wagon Hill (OR4 and OR5). Sample sites above the dam: College Brook (CB1, CB2, CB3, CB4); Mill Pond (MP1, MP2, MP3).
To meet these objectives, sediment samples were collected from Mill Pond, Oyster River, and College Brook on and near the UNH campus. All sample locations are shown in Figure 1. These sediment samples were collected from the waters’ edge up to 10 cm below the surface with small, clean plastic scoops and stored in double bags. Samples were collected in triplicate at each site on July 17, August 2, and September 5, 2023. Additional samples were collected from the Oyster River and two sites at Mill Pond on July 13, and a singular set of samples was collected from Wagon Hill near the mouth of the Oyster River on July 31. Sampling was a challenge in this project because initiating sampling in a stormy summer was difficult. Heavy rainfalls in June and July 2023 required sample collection on dry days where it also had not rained heavily for two or three days beforehand. This was important because heavy rain could make sites inaccessible or create surface disruptions not necessarily representative of the system as a whole. Eventually, I obtained samples in mid-July, early August, and early September, allowing for a time series of data through summer 2023.
Samples were then processed in a lab on the UNH campus through freeze drying, which removes all the water from the sample. After processing, the sediment samples have a powderlike texture. I then analyzed the processed samples for total mercury with the Milestone Direct Mercury Analyzer. This instrument heats the sample to release the mercury and collects and concentrates the mercury on a gold trap, where it is then reheated and released for measurement.
The amount of organic matter in each sample was estimated through a technique that involved measuring the mass lost upon ignition in a heated furnace. Here, the processed sample is weighed out in crucibles, which are then combusted in a furnace at 450°C for four hours. After this combustion, the inorganic sandlike component of the sediment remains, and the samples are cooled and then weighed again. The mass differences between the original sample and the heated sample provided an estimation of the percentage of organic matter in the sample. From these data, the estimated amount of organic carbon could be calculated.
Characterizing both mercury and organic content provides an understanding of how mercury is hosted in the watershed, either with organic matter or merely on sediment. In an uncontaminated estuary, mercury tracks through the watershed with organic carbon, while in a locally contaminated watershed, mercury cycles through the watershed and does not demonstrate robust relationships to organic carbon (Seelen et al., 2021). In the measurements for my SURF and undergraduate thesis, I found the relationship between mercury and organic carbon differs in contaminated and uncontaminated estuary sites.
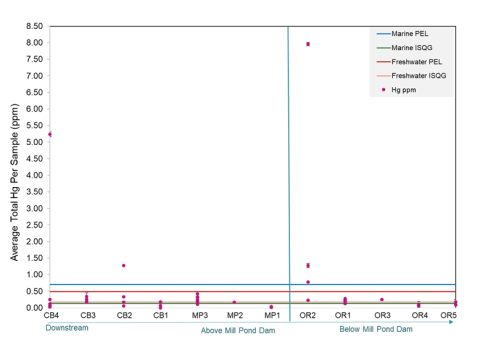
Figure 2: Total mercury at each site in the Oyster River-Mill Pond watershed. The lines on the chart indicate each marine or freshwater sediment quality guideline. The ISQG indicates where negative effects on the ecosystem from the mercury are possible. The PEL indicates where negative effects on the ecosystem from the mercury are possible.
Results and Discussion
As of March 2024, approximately fifty-three sediment samples collected in the summer 2023 campaign have been fully processed and analyzed for percent lost on ignition and total mercury analysis.
The data analysis focused on comparing the sediment samples’ total mercury concentrations with the sediment quality guidelines recommended by the National Resource Council (NRC) of Canada. The NRC of Canada issues two guideline concentrations for marine and freshwater sediment: the interim sediment quality guidelines (ISQG) and the probable effect level (PEL). The ISQG indicates when the mercury in an area will begin to cause adverse effects to the ecosystem. The PEL represents where it is probable for the organisms in the ecosystem to display negative effects of the toxin. Freshwater and marine sediments have different concentration thresholds for the ISQG and PEL. The ISQG recommendations were the focus of my data analysis, and most sediments in the watershed contain concentrations above this standard. Several samples also exceeded the PEL.
The mercury concentrations in the sample sites below the dam were variable both within each site and throughout the Oyster River, as shown in Figure 2.
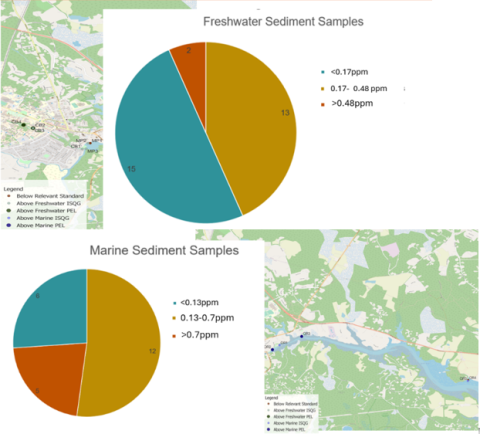
Figure 3. Breakdown of above and below dam sediment samples, showing those above each sediment quality guideline and where they were collected.
The mercury concentrations in the Oyster River at Old Landing, just below the Mill Pond Dam (site OR2), had total mercury concentrations ten times greater than the marine PEL, with all contents at this site in all but one sample measuring above the PEL. The other sample sites in the Oyster River at Old Landing (OR1) and the site at Jackson’s Landing (OR3) also had elevated total mercury amounts, with all studied Oyster River sites close to the Mill Pond Dam demonstrating mercury contents above the ISQG. The sites at Wagon Hill (OR4 and OR5), farther downriver, had lower total mercury concentrations, with some samples at OR5 containing mercury concentrations above the ISQG.
For the sample sites above the Mill Pond Dam, the College Brook sites contained varying amounts of total mercury within individual sample sites and the area as a whole. A site under the Memorial Union Building (CB2) had some concentrations of mercury exceeding the freshwater PEL. A site farther upstream from the Memorial Union Building (CB4) also contained mercury samples that exceeded the PEL.
The remaining College Brook sites, CB1 and CB2, as shown on the sample site map, had mercury concentrations above the freshwater ISQG. The three sites at Mill Pond (MP1, MP2, MP3) had relatively lower mercury amounts, with few samples exceeding the freshwater ISQG at sites MP2 and MP3. The breakdown of the number of samples exceeding the relative ISQG and PEL both above and below the dam is displayed in Figure 3.
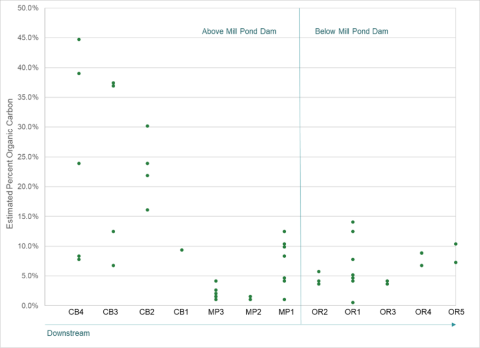
Figure 4. Estimated organic carbon at each sample site, calculated from percent of organic matter loss on ignition. The X axis contains the site code for each site: CB (College Brook); MP (Mill Pond); and OR (Oyster River).
The amount of estimated organic carbon in these samples was also variable, more so above the dam than below it, as seen in Figure 4. In the samples below the dam in the Oyster River, there was no relationship between the estimated amount of organic carbon and mercury when all the samples were considered. When just the samples below the marine PEL were considered, there was some relationship between total mercury and organic carbon. In the freshwater sediments, there was no relationship between total mercury and organic carbon when all the samples, or just the samples below the PEL, were considered. These relationships are displayed in Figure 5.
My research findings show that the amount of total mercury in the Mill Pond Dam watershed is variable within individual sample sites and between the studied sample sites. When evaluating the links between mercury and organic carbon, the relationship in the marine sediments below the dam is characteristic of uncontaminated sediments, but the lack of relationship between organic carbon and total mercury in the freshwater sediments is a characteristic of a local or legacy contaminated site.
The high total mercury concentrations above the PEL were unexpected, as were some of the sites with low concentrations recorded in Mill Pond, given findings reported for cores studied by Miller (2020). It is possible that the low concentrations in one of the Mill Pond sites were because of the heavy rainfall in summer 2023 that may have reallocated surface sediments near the shore where sampling took place.
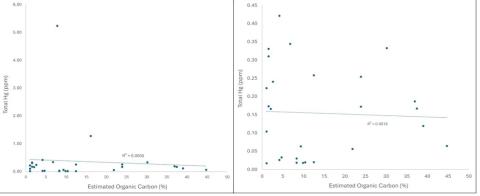
Figure 5. Left; relationship between total mercury and estimated organic carbon in all freshwater sediment samples. Right; Relationship between percent organic carbon and total mercury in the samples below the freshwater PEL.
Taken as a whole, the study findings further indicate that there is mercury present variable amounts in the sediments above and below the Mill Pond Dam, and that many of the sediments above the dam bear the characteristics of locally contaminated sediments (Seelen, 2021).
I presented this research at the meeting of the Northeast Section of the Geological Society of America in March 2024 (Ward et al., 2024). I will share this presentation and the thesis I am completing this spring with the Town of Durham with the hope that it will be used to guide sediment removal decisions. Planned sediment removal will prevent sediments from flowing downstream into the Great Bay, ultimately preventing its contamination.
Thank you to Julie Bryce and Maria Florencia Fahnestock for your mentorship and mercury expertise. Thank you to the Hamel Center for Undergraduate Research—and Mr. Dana Hamel in particular—for the funding for this project.
References
Castoldi, A. F., Coccini, T., & Manzo, L. (2003). Neurotoxic and Molecular Effects of Methylmercury in Humans. Reviews on Environmental Health, 18 (1). https://doi.org/10.1515/reveh.2003.18.1.19
Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. (2002). http://www.popstoolkit.com/Tools/SitePrioritization/Files/Guidelines/Se…
Dandurant, K. (2022, March 8). Durham votes to remove Mill Pond dam with 74% of vote and large turnout. Seacoast Online. https://www.seacoastonline.com/story/news/local/2022/03/08/durham-nh-mi…
Driscoll, C. T., Mason, R. P., Chan, H. M., Jacob, D. J., & Pirrone, N. (2013). Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environmental Science & Technology, 47(10), 4967–4983. https://doi.org/10.1021/es305071v
Miller, H. (2020). Contributing to the Field of Dam Removal Science: Analyzing Sediment Characteristics in Mill Pond and Sawyer Mill (2020). Inquiry Journal, 7. https://scholars.unh.edu/inquiry_2020/7
New Hampshire Department of Environmental Services (2024). Projects: Planned, Completed and Under Consideration. https://www.des.nh.gov/sites/g/files/ehbemt341/files/documents/2020-01/…
Seelen, E. A., Chen, C. Y., Balcom, P. H., Buckman, K. L., Taylor, V. F., & Mason, R. P. (2021). Historic contamination alters mercury sources and cycling in temperate estuaries relative to uncontaminated sites. Water Research, 190, 116684–116684. https://doi.org/10.1016/j.watres.2020.116684
Ward, J., Bryce, J., & Fahnestock, M.F. (2024). Sediment Mercury Above and Below the Head-of-Tide Mill Pond Dam in Durham, NH, Geological Society of America Abstracts with Programs. Vol. 56, No. 1. doi: 10.1130/abs/2024NE-397667.
Author and Mentor Bios

Oyster River in Durham , NH.
Originally from South Kingstown, Rhode Island, Julia Ward will graduate in May of 2024 with a Bachelor of Science degree in Environmental Science: Geosystems. She conducted this research through a Summer Undergraduate Research Fellowship though the Hamel Center for Undergraduate Research and continued work on this project to complete an undergraduate thesis. Julia was drawn to the project by her interest in geochemistry and was excited to work on a project that involves something local. She learned that research is a process that has unexpected challenges, but especially enjoyed the fieldwork aspect of this project in being outside and seeing the field sites. Julia enjoyed connecting the fieldwork with the analysis of this project, collecting the samples that she ended up working with for the next six months. She decided to publish in Inquiry because she wanted to share the results of her research in a place where people could read it and write something for a more general audience. She plans on finding employment in her field after graduation and this research experience has given her valuable skills towards this goal.
Dr. Julie Bryce is a professor of Earth Sciences and serves as undergraduate coordinator for the Department of Earth Sciences. Her primary research interests lie in the application of trace elemental and isotope geochemistry to time and track processes in Earth surface and deep Earth environments. In addition to studying how elements and isotopes cycle through the Earth system, Dr. Bryce also has projects collaboratively with faculty in other disciplines.
Copyright 2024, Julia Ward
