Is It Hot Here? How Increased Water Temperature Impacts Tunicates on a Physiological Level
When you think of marine species that live in the Gulf of Maine, a tiny invertebrate attached to the side of a dock may not be the first one that comes to mind. However, tunicates have a powerful influence on other species within their environment. This is especially true in the Gulf of Maine, where they have been found on the side of boat docks, tangled up on lobster traps, and attached to oyster and mussel beds. As research about these animals expands, we see their direct impact on their environment. Increasing amounts of invasive species of tunicates can negatively impact the organisms around them.

Figure 1. Photo of large adult tunicate colonies.
Tunicates suffocate mussels by growing over and completely encapsulating them, which reduces their growth and ultimately the amount of meat harvested for aquaculture. If there aren’t any mussels to farm, there aren’t animals to harvest, so the farmers don’t make as much money and they aren’t too happy. By suffocating and decreasing the mussel population within the Gulf of Maine, tunicates are clearly having a negative impact on aquaculture in the region.
Tunicates do hold value within the ecosystem because they act as ocean purifiers by consuming bacteria (Fatherree, J. (2015)). They are also an important part of the food web because they consume phytoplankton and various bacteria within the water column (Fatherree, J. (2015)). Though these are some positive impacts, tunicates alter the aesthetics of docks by simply existing within marinas. Increasing numbers of slimy orange tunicates in marinas mean more of them show up on the boats that dock there. This therefore forces boat owners to clean their hulls more often.
A better understanding of how these species impact ecological systems and the aquaculture systems around them is crucial for the health of the community and the overall planet as we combat invasive species and climate change. Many non-native species thrive in warmer waters, and tunicates are no exception. The Gulf of Maine is warming faster than 99% of the ocean (Poppick, 2018). It is especially susceptible to warming because of its location at the meeting point of major ocean currents and its relatively shallow depth. Therefore, with gracious funding from a Summer Undergraduate Research Fellowship (SURF) through the Hamel Center for Undergraduate Research, I studied how rising water temperature influences the presence of a non-native species of tunicate, Botrylloides violaceus, within the Gulf of Maine.
Those Orange Blobs Are Alive?
Yes, they are alive! Tunicates are soft-bodied marine invertebrates found in large quantities throughout various oceans. Tunicates are sea squirts, and they all have a rubbery type of covering and two siphons (tubes/filters) to draw water in and out of the body. My study species, Botrylloides violaceus, can be multiple colors, the main one being orange, but also red, orange, yellow, or dull purple. Adult colonies have small “holes” on their bodies called zooids (zoh-eds) (Ghiselin, 2023). Zooids are embedded within a transparent layer on the body and connected by a network of blood vessels. These blood vessels terminate in small sac-like structures around the outer edge of the colony (Ghiselin, 2023). Each zooid has a stomach, intestines, and heart. In other words, each zooid has a heartbeat, which means that you can cut down the middle of a tunicate colony and each side will still be alive!
Where Can You Find Them?
Geographically, the orange sheath tunicate, though native in the northwest Pacific from southern China to Japan, is starting to be found more often in the coastal waters of North America. The species was first observed in the Gulf of Maine in the early 1980s (Fatherree, J. (2015). In addition to my study species, there are at least three other non-native, and several native, species of colonial tunicates. These non-native species have come into this new environment and adapted so well that they have distributed themselves all over the New England coast and often outperform other species (Fatherree, J. (2015). The most common place to find this species is within the subtidal zone and periodically in protected areas within the intertidal zone. They like to attach themselves to any solid surface! This includes sides of docks, buoys, lobster cages, rocks, kelp, mussels, and more. They can wrap around corners, and even fold over on themselves. They are masters at reproducing as well. All colonial tunicates reproduce asexually (a mode of reproduction in which an offspring is produced by a single parent) through “budding” and reproduce sexually from fertilized eggs that eventually develop into larvae (Ghiselin, 2023). Whether sexually or asexually, the process begins with the parent colony dying off and, in the process, releasing as many babies into the water as fast as they can. These babies are visible to the naked eye and tend to swim around for ten to fifteen minutes until they find a surface to grow on. This type of reproduction therefore makes them spread easily because of the large number of babies produced.
How Do Non-Native Species Impact the Gulf of Maine?
A species’ distribution can depend on various biotic and abiotic factors, and its establishment is often dependent on several variables, including its response to local environmental conditions (Gomes-Filho et al., 2010). Typically, Maine beach water temperatures peak in the range of 17 to 20°C and a low between 2 and 5°C. Many species within an ecological system learn to live next to and with one another, and to survive, all organisms must learn to adapt to their surrounding environment (Zhan et al., 2015). Non-native species directly impact the invaded ecosystem of that community. Botrylloides violaceus have outcompeted native tunicate species in the area, and they are a crucial part of the food chain (Zhan et al., 2015); a decrease or increase in the native species population alters the food web.
The orange sheath tunicate was my chosen study species because their physiological response to increasing temperatures and heat waves is yet unresolved. To address this research gap, I partnered with graduate student Madison Hurley from the Department of Biological Sciences at the University of New Hampshire (UNH) on the response of B. violaceus to thermal stress. I built my research off her study, which focused on how tunicates’ heart rates change in response to increased water temperature. My specific focus was to investigate how dissolved oxygen concentrations were impacted by heat waves. Oxygen concentrations are directly related to the organism’s overall health, and its consumption is a measure of oxidative stress. I hypothesized that dissolved oxygen would decline over the seventy-two-hour trial, especially at higher temperatures. This therefore would mean that the tunicates’ health would decrease as the temperatures increased (Dijkstra et al., 2007). This statement came from known knowledge on how dissolved oxygen concentrations change in higher temperatures as well as previous studies done on this species of tunicate by my mentor, Dr. Jennifer Dijkstra. I hoped to get a general understanding of how tunicate samples physically changed over time with every dissolved oxygen reading.
Methodology
My study was carried out through multiple four-day trials over three months in the summer of 2023. I started a trial by collecting eight colonies (all roughly the size of a thumb) from the boat docks at Wentworth Marina in New Castle, New Hampshire. We hung off the docks and gently peeled tunicates of similar size from the surfaces of buoys, mussels, seaweed, and even the dock itself. We then transported them in a cooler full of the same water from their environment back to the coastal marine lab at the Judd Gregg Marine Research Complex nearby. We helped the tunicates adhere to glass slides by using wool string to tie the edges of the colony lightly to the slide. Once they were tied, we placed them gently in their desired treatment tanks, and came back the next day to start the acclimation process.
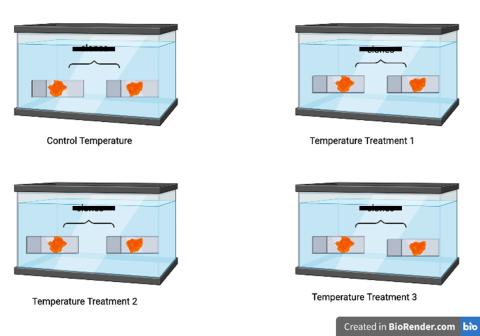
Figure 2. Visual of how the tanks were set up, one animal for testing heart rates, the other testing dissolved oxygen concentrations.
There were four treatment temperatures: control tank (18°C), treatment 1 (22°C), treatment 2 (25°C), and treatment 3 (28°C). The control tank temperature represented the average ocean temperature in the Gulf of Maine during the summer months. We had to let the tunicates acclimate to the change in water temperature by slowly increasing their ambient water temperature to the desired trial temperature. If we placed them in tanks in water with a drastically different temperature from their ambient water, they would experience heat shock, which would lead to stress and death. We slowly acclimated them to the desired treatment temperatures by using water heaters to increase the ambient temperature in the tank by one degree every hour for eight hours. Once a tank reached a temperature that was two degrees lower than the desired treatment temperature, we stopped the acclimation process and let the tunicates acclimate to that temperature overnight. For example, for treatment 1, the water temperature started at 18°C, the next hour we raised it to 19°C, and so on. Once treatment 1 hit 20°C, two degrees lower than the desired treatment temperature of 22°C, we stopped acclimating that tank and continued with the other tanks until they reached two degrees lower than their desired temperatures. We then let the tunicates sit overnight in those temperatures to slowly acclimate them over that period.
The next day, the seventy-two-hour warming trial began! We got to the lab an hour and a half early, turned up the heaters to warm the water the last two degrees, waited until we saw that it hit the desired trial temperature, and started our readings at hour zero. This hour represented how they initially reacted to the temperature shift, and then how they would (or wouldn’t) acclimate to that temperature. To obtain a heart rate reading, we placed the tunicate in a petri dish filled with the same water from the tank it came from. Using an Olympus Infinity microscope, we zoomed in and searched for three separate heart rates on the animal, recorded beats per minute, and averaged the three to get an overall reading. When they are viewed under the microscope, a whole new world comes to life! You can see the intricate details of their blood vessels as well as many different heart rates within the zooids. The “blood” within these animals has many different cells flowing through it, and you can see these individual cells moving at different speeds throughout the tunicate.
To take a dissolved oxygen reading, we first took a blank reading (one with no animal inside) and then a non-blank (one with the animal inside). We used Vernier Probes along with the Vernier Graphical Analysis program to collect this data. We put the probes in an encapsulated glass tube with a stir bar and cap on top to seal it. We then placed each tube in a water bath with the same water from the tank it came from, turned on the stirrer, and let it run for two minutes before collecting the data we would use for our analysis. We did this to eliminate as many errors as possible. There was one water bath containing an animal and one without. We then kept the stirrer running, restarted the timer, and ran it for a one-minute period. Once the minute was up, we hit “Stop” and the data we needed was presented on the computer screen.
We collected both the heart rate and the dissolved oxygen readings at time zero, six, twenty-four, forty-eight, and seventy-two hours throughout the trial. Hour zero represented the start of each trial, almost like a control reading. This process counted as one trial done, and we repeated the process for ten four-day trials.
Preliminary Results and Conclusions
Our results showed that Botrylloides violaceus did not respond well to increased heat and their heart rates majorly climb and then just as quickly decline. The heart rates initially increased when the animals became stressed because of the change in temperature, and they decreased once they couldn’t handle being in that temperature anymore. This resulted in a slow death, when sometimes their hearts stopped beating completely. The temperature that caused this reaction consistently was the treatment with the highest temperature (tank 3). Within each trial, animals in treatment 1 and 2 had an equal chance of either adapting to the temperature or dying because of it.
In addition, the dissolved oxygen data showed that on average, the control tank had higher concentrations of dissolved oxygen compared with the treatment tanks. This was not surprising, because warmer water has less dissolved oxygen concentrations. As temperature increases, it causes the gas and water molecules to gain more energy and break the weak molecular interactions between water and oxygen molecules (Pershing et al., 2015). Furthermore, those colonies exposed to warmer temperatures experienced stress and therefore, may take up greater amounts of dissolved oxygen. Moreover, though sizes of colonies were not measured, colonies placed in higher temperatures were visually deteriorating and dissolving by the end of the trial, which clearly indicated poor health.
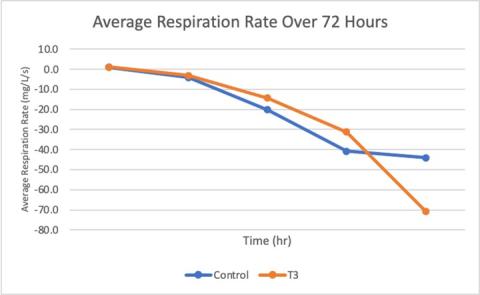
Figure 3. Comparing the Average Respiration Rate over a trial period between Control (18°C) and Treatment 3 (28°C). Each dot represents when a reading took place (hours 0, 6, 24, 48, and 72).
If warmer water temperatures and heat waves, as predicted for the Gulf of Maine, become more consistent, they could negatively affect this non-native species and other ectothermic (individuals whose body temperature is the same as ambient conditions) species. If dissolved concentrations become alarmingly low, the water would become hypoxic, meaning that the environment has low or depleted oxygen levels (Pershing et al., 2015). With a decrease in dissolved oxygen, there is a probability that the Gulf of Maine would become more acidic with the warming temperatures. While further research would need to be done, this could possibly decrease species diversity drastically. This would drastically impact the food web, which would impact not only tunicates, but every animal that relies on the Gulf of Maine for nutrients or habitat of any kind. While the consequences of a declining tunicate population are currently unknown, it would likely help enhance mussel and oyster aquaculture because the farmers wouldn’t have to clean their lines or scrub their surfaces during harvest. Furthermore, mussel and oyster populations could increase because they wouldn’t be competing with the tunicates for food sources in the water column (Rybovich et al., 2016).
It can be seen in the figure above that while the control had a similar decline within the first forty-eight hours, the decline rate was slower and ultimately slowed down toward the end of the trial. However, the treatment 3 animal had a faster respiration rate decline and did not level out.
Personal Takeaways
The Summer Undergraduate Research Fellowship (SURF) program not only taught me independent lab skills but also life skills. I lived on my own for the first time, navigated my own independent research, and learned how to solve problems more intently. This experience has been a stepping stone by allowing me to perform independent research in a lab that I might not have become involved with otherwise.
Educationally, this research has given me a better understanding of tunicates and their role in marine ecosystems. Before this, I had general knowledge of the species, but doing my own research on them allowed me to truly comprehend their biology and influence on their habitat. As Madison and I worked more regularly with tunicates, I grew a deeper appreciation for the species and therefore became more connected to my research. I quickly learned that Botrylloides violaceus are not the only crucial invasive species in the New England area. There are invasive shrimp, bryozoans, mollusks, and various other species. Within my field of marine biology, understanding the way that this species interacts with the native and/or other invasive species directly ties into understanding the ecology of marine habitats and what further research could look like.
Now that I am in my senior year, I am thinking about future careers and what I am going to be looking for within a job. The SURF program has helped me become passionate about research. I would love to find a research-oriented position after graduation, or even pursue a master’s degree that focuses on research.
The advice that I would give for future researchers is to keep your head up and keep problem-solving. While that may sound simple, it truly does make all the difference in this field. There were times when equipment didn’t work, I made a mistake within the procedure, or things didn’t seem to be going my way, but those times are when the most growth happens. I learned that things won’t always go my way, I will make mistakes, and I will have to rework procedures, but having the mindset that those moments are only opportunities for growth is how one will swim rather than sink within the field of research science.
First, I would like to thank Madison Hurley for guiding me along each difficult part of this project, encouraging me, and being a great friend throughout the journey of my study. Furthermore, I am grateful for Jennifer Dijkstra and the help she extended to me by being my adviser. Additionally, I’m thankful that Brittany Jellison and Nate Rennels were willing to support me by providing amazing lab equipment and lab space. Without their support and guidance, I wouldn’t have been able to complete my study or have data to discuss. I also want to extend a huge thank-you to my parents, close friends, and boyfriend for listening to me talk nonstop about my project and data for three months straight. I couldn’t be more thankful for their continued support and incredible listening skills. Finally, the project wouldn’t have been possible without the funding from the Hamel Center for Undergraduate Research through the Summer Undergraduate Research Fellowship — with specific support from Mr. Dana Hamel and the J. Raymond Hepler Endowed Fund — to which I owe this unforgettable experience and guidance for my future.
References
Dijkstra, J., Harris, L. G., & Westerman, E. (2007). Distribution and long-term temporal patterns of four invasive colonial ascidians in the Gulf of Maine. Journal of Experimental Marine Biology and Ecology, 342(1), 61–68. https://doi.org/10.1016/j.jembe.2006.10.015
Fatherree, J. (2015). An Introduction to Tunicates. Reefs.Com. https://reefs.com/magazine/an-introduction-to-tunicates/
Ghiselin, M. (2023) Tunicate | Anatomy, Habitat & Adaptations | Britannica. (n.d.). Retrieved November 25, 2023, from https://www.britannica.com/animal/tunicate
Gomes-Filho, J. G. F., Hawkins, S. J., Aquino-Souza, R., & Thompson, R. C. (2010). Distribution of barnacles and dominance of the introduced species Elminius modestus along two estuaries in South-West England. Marine Biodiversity Records, 3, e58. https://doi.org/10.1017/S1755267210000461
Pershing, A. J., Alexander, M. A., Hernandez, C. M., Kerr, L. A., Le Bris, A., Mills, K. E., Nye, J. A., Record, N. R., Scannell, H. A., Scott, J. D., Sherwood, G. D., & Thomas, A. C. (2015). Slow adaptation in the face of rapid warming leads to collapse of the Gulf of Maine cod fishery. Science, 350(6262), 809–812. https://doi.org/10.1126/science.aac9819
Poppick, L. (2018, November 12). Why is the gulf of maine warming faster than 99% of the ocean? Eos. http://eos.org/features/why-is-the-gulf-of-maine-warming-faster-than-99-of-the-ocean
Rybovich, M., La Peyre, M. K., Hall, S. G., & La Peyre, J. F. (2016). Increased Temperatures Combined with Lowered Salinities Differentially Impact Oyster Size Class Growth and Mortality. Journal of Shellfish Research, 35(1), 101–113. https://doi.org/10.2983/035.035.0112
Zhan, A., Zhang, L., Xia, Z., Ni, P., Xiong, W., Chen, Y., Douglas Haffner, G., & MacIsaac, H. J. (2015). Water diversions facilitate spread of non-native species. Biological Invasions, 17(11), 3073–3080. https://doi.org/10.1007/s10530-015-0940-1

Author and Mentor Bios
Originally from Rochester, New York, Melanie Burley will graduate in May 2024 with a bachelor of science degree in marine biology. She serves as the business manager of Off the Clef, one of the co-ed a cappella groups on campus, and is part of InterVarsity Christian Fellowship. She began her research project while working with graduate student Madison Hurley, who was also doing tunicate research at the time and introduced her to the SURF program through the Hamel Center for Undergraduate Research, which funded Melanie’s project. Melanie found it interesting to learn more about the invertebrates that she had never even noticed before being introduced to them, and about how her study species have been negatively impacting the aquaculture community. During research, Melanie had fun collecting the animals off the docks and completing trials, making her feel confident in the lab. Additionally, she learned a lot about research in general, and how to work through issues and problems effectively. Her overarching career goal is to continue being a part of hands-on research or work at a job in marine science. Melanie would love to be part of a growing group of scientists who want to make a difference in their communities for the benefit of others and the marine world.
Jennifer Dijkstra is a research associate professor at the Center for Coastal and Ocean Mapping, having joined the University of New Hampshire in 2001 as a graduate student. She specializes in the study of marine species communities, employing innovative methods, techniques, and technologies to forecast their spatial distribution and explore the reasons behind, as well as the implications of, their distribution changes over time. This study evolved when the author showed interest in tunicates and wanted to gain more experience through independent research. Dr. Dijkstra has mentored undergraduate students before, but this is her first time mentoring an Inquiry author. She says the experience has been wonderful. “Mel is very hardworking and is interested in all aspects of the study. It’s obvious she has a keen interest in and enthusiasm for marine species.”
Copyright 2024 © Melanie Burley
