Influence of Dissolved Oxygen on the Degradation and Transformation of NEtFOSE within a Secondary Wastewater Treatment Bioreactor System
Per- and polyfluoroalkyl substances, more commonly known as PFAS, are a large family of synthetic compounds that have been used in commercial and industrial settings since the 1940s. These substances are commonly found in municipal products including detergents, waterproof clothing coatings, and coatings on nonstick cookware due to their thermal stability and ability to repel water and oil. These persistent organic pollutants, some of which have been phased out or banned in certain countries or states, can exhibit toxicity effects such as immunotoxicity, genotoxicity, reproductive toxicity, neurotoxicity, and carcinogenicity. They have also been shown to interfere with thyroid function in humans (Zhang et al., 2020).
PFAS have proven to be problematic because they don’t break down in the environment, or only partially degrade to other PFAS. There are over 5,000 substances, and they have been detected in even the most remote environments in the world, such as the high Arctic (Rhoads et al., 2008). These pollutants are also found in the blood of 99% of Americans (Environmental Working Group, 2018).

Figure 1. Alexis Eaton spiking wastewater samples with NEtFOSE. Click to enlarge.
With funding from a Summer Undergraduate Research Fellowship (SURF) at the University of New Hampshire, I studied N-ethyl perfluorooctane sulfonamido ethanol (NEtFOSE), a PFAS historically used in paper manufacturing and a precursor (or degradation product) to other per- and polyfluoroalkyl substances. The compound itself is extremely toxic; however, it degrades into even more harmful and bioaccumulative compounds including perfluorooctane sulfonic acid, also known as PFOS (Rhoads et al., 2008). As the only company in the US to incorporate the compound into its products, 3M phased out PFOS production beginning in 2002; however, it is still commonly detected in environmental media including atmospheric samples (Zhang et al., 2020). Furthermore, NEtFOSE, while not in use in the US, is still incorporated into products manufactured overseas (Zhang et al., 2020). NEtFOSE has made its way into surface water bodies, wastewater, drinking water, and soils. It is vital that the behavior of this compound is studied in order to understand its fate and protect human and environmental health from exposure.
For my research project, I studied how dissolved oxygen levels influence the degradation and transformation of NEtFOSE during wastewater treatment. The study took place at the Exeter, New Hampshire, Wastewater Treatment Plant. By collecting wastewater samples spiked with NEtFOSE from both aerobic and anaerobic zones of a secondary treatment basin in Exeter’s larger wastewater treatment system, I was able to observe the biotransformation pathways of the compound in each environment and make meaningful comparisons. Outcomes from this research can inform scientists in targeting the destruction of PFAS compounds in wastewater because it will help them understand which metabolites, meaning intermediate or end products, will likely be present based on whether dissolved oxygen is present. If scientists can then target the destruction of these metabolites in the right places in the wastewater treatment system, the wastewater discharged from the plant will be less likely to contaminate water bodies with PFAS.
Membrane Conceptualization and Design

Figure 2: Membrane bioreactors filled with decanted wastewater. Click to enlarge.
The first stage of my research project was spent designing and building the membrane bioreactors that would be used to hold the wastewater we collected from the secondary treatment basin of Exeter’s treatment plant and then spiked with NEtFOSE. A bioreactor is a system that supports a biologically active environment. The term membrane refers to the material that encases this environment. I worked with Kellen Sawyer, one of the research technicians in Professor Paula Mouser’s lab, to construct the systems. My mentor, Dr. Mouser, is a professor of civil and environmental engineering at UNH.
I used a food-grade cellulose dialysis membrane, which is a semipermeable material that can be thought of as a “sausage casing.” I used aluminum flat stock to press these dialysis membranes closed and attached them with the appropriate hardware. It was important that the membranes were tightly sealed so PFAS in our spiked wastewater didn’t leach out and contaminate the surrounding wastewater when we placed the bioreactors in the aerobic and anaerobic basins of the treatment plant.
We created two bioreactors with three membranes in series (Figure 2). The pore size of the membrane determines what types of molecules can pass through the membrane and which others are trapped inside. Molecules larger than the pores, which were 24 angstroms in diameter (including PFAS), cannot pass out of the membrane and contaminate the water in the secondary treatment basin, but small molecules like oxygen, pure water, and nutrients can do so freely. The ability of oxygen and nutrients to pass through is what allows the wastewater in the membrane to be representative of the surrounding wastewater. The bioreactor serves as a microcosm of the secondary treatment basin; it allowed us to study this concept in a safe way because the bioreactor prevented PFAS inside the membranes from contaminating the surrounding wastewater in the treatment basin.
Preliminary Experiments
The second step in my research was to ensure that the sludge within the wastewater that we had collected from the secondary treatment basin and put inside the membranes remain healthy as time progressed. This would ensure that the wastewater inside the membranes would be an accurate representation of the surrounding system. The term “healthy sludge” refers to semisolid organic matter within wastewater that is rich in microbes and bacteria which break down pollutants. Without these microbes, contaminants cannot be removed, and the wastewater would be unsafe to discharge into water bodies.
In order to test if the sludge would remain healthy, we filled the two membrane bioreactors with wastewater and placed them in a secondary anoxic wastewater basin, which was a mix between an aerobic and anaerobic basin; we regarded this as day zero. We collected samples from two of the individual membranes per day on day one, day two, and day five. We also collected regular wastewater samples (that were not inside the bioreactor) from the secondary treatment basins on each of these days. With each of the samples, I conducted Total Suspended Solids (TSS) and Volatile Suspended Solids (VSS) analyses.
The idea behind the experiment was that if microorganisms within the membranes were dying due to environmental stressors, such as a lack of nutrients, the biological cells would rupture, and we would see a change in the solids concentration inside the bioreactor. If the microorganisms within the membrane remained active and healthy, the solids concentrations would match that of the samples taken from the treatment basin. We expected the TSS and VSS values for the wastewater samples taken from the treatment basin itself to remain relatively constant over time. Our ultimate hope was that the TSS and VSS values for the wastewater samples inside the membranes would also remain constant over time. If these values decreased or increased drastically as time progressed, it would imply that the sludge within the membranes would not be healthy enough for bacteria to actively degrade and transform NEtFOSE during the next phase of our research. This preliminary experiment would also allow us to ensure that the membranes themselves do not leak or dissolve when placed in a secondary wastewater system, and that a healthy biological environment exists within the membranes.
In regard to the results of this preliminary study, both the TSS and VSS results show that solid concentrations within the basins were higher than the solid concentrations in the membranes for day one and day two. Data from day five suggests that there was an increase in biomass, or solids concentrations, within the membranes, which was greater than solid concentrations in the basin itself. We were able to conclude that the loss of suspended solids that occurred during the first two days was due to the consumption of organic matter within the membranes. The gain in biomass on day five suggests that the membrane systems were “trying” to be in equilibrium with the surrounding wastewater.
Ultimately this experiment provided some important considerations for our next experiment. The first was that solids began sticking to the outer surface at day five, suggesting some biofouling was occurring that could reduce passage of oxygen and nutrients into the membrane. The second was that solid concentrations were different in each membrane, which could allow for differing levels of breakdown. With this information, we decided that it would be best to decant, or allow for large solids from the wastewater to settle out, so that the wastewater that we used in the membrane bioreactors was primarily composed of smaller, more uniform biological cells.
NEtFOSE Experiment
In order to assess how the presence of oxygen affects the breakdown and transformation of NEtFOSE, graduate student Lindsay Guertin and I let the solids settle out from the wastewater before adding it to the membrane bioreactor system. We then spiked the wastewater by creating a stock solution with the NEtFOSE and placing it into the membranes with the decanted wastewater. One of the membrane bioreactor systems was deployed in the anaerobic basin, and the other was placed in the aerobic basin, each for three days. The bioreactors were attached to the railings around the basin with a split ring and eye bolt. A chain was attached so the membranes could hang down ten feet below the surface of the water.
Lindsay and I conducted an ATP assay with the samples we collected in order to assess the health of the wastewater in the membranes. An ATP assay is a procedure that measures cell viability based on detection of ATP, which is an organic compound that provides energy to living cells. The more ATP, the more energy is being used or generated by bacteria in the wastewater. The ATP assay yielded an increase in relative light units, which implies that by removing the solids before putting them in the membranes, we didn’t allow for plant “grazers” like protozoa to be included within the system. Without the “grazers,” the bacteria in the membranes grew differently than they would have in an open wastewater system. Therefore, the results of this study can't be generalized to regular sludge-containing wastewater, but they are still indicative of the role that oxygen and bacteria play in PFAS degradation.
In contrast, we observed a lowering of ATP in the aerobic membranes, which could be related to the speed at which bacteria used up nutrients in the membranes, then began to die. Breakdown of wastewater compounds generally occurs much quicker under aerobic conditions as opposed to anaerobic conditions.
Preliminary Findings
We then sent Alpha Analytical Laboratory, a local environmental testing laboratory, three samples of spiked wastewater from the bioreactors in each basin. We also sent a sample of wastewater that hadn’t been spiked with NEtFOSE, as well as a spiked sample that had not been placed in the basin for the three days. These served as negative and positive controls, respectively. The lab used an Environmental Protection Agency (EPA) standard method to analyze for 24 important PFAS at the part per trillion level in these samples. The data proved to be very promising in showing a distinct difference in how NEtFOSE breaks down under anaerobic versus aerobic conditions. Figure 3 shows how PFAS compound concentrations compared in the different environments.
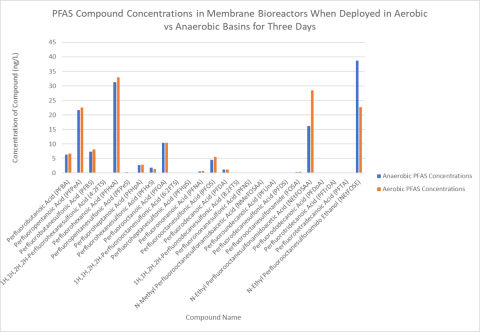
Figure 3. PFAS compound concentrations in membrane bioreactors when
deployed in aerobic vs anaerobic basins for three days. Click to enlarge.
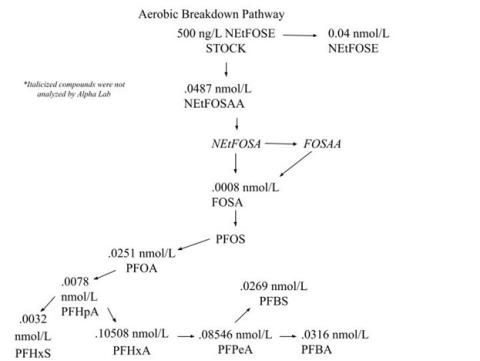
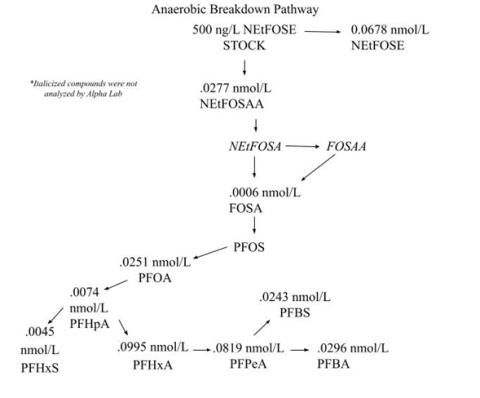
Ultimately, the results suggest that the only significant change in NEtFOSE breakdown between the anaerobic and aerobic environments relates specifically to NETFOSAA, another PFAS compound. Under aerobic conditions, most of the NEtFOSE transforms into NEtFOSAA; whereas, under anaerobic conditions, a smaller concentration of NEtFOSE breaks down into NEtFOSAA, and a majority of the NEtFOSE remains intact. So little research has been done on these compounds that it is unknown whether one is more toxic to humans and animals than the other; therefore, this study did not allow me to classify whether an aerobic or anaerobic environment is more effective at reducing the toxicity of wastewater. Some biotransformation products may accumulate under anaerobic conditions because their rate of degradation is slower than aerobic conditions. If those transformation products are more toxic, this may present a human health concern.
Next, I compared Alpha’s results from the anaerobic and aerobic bioreactor samples to the control samples. I had to take into account the molar masses of each of the individual PFAS compounds in order to truly see whether or not there was a difference in the amount of each compound present in the membranes versus the controls. This confirmed that the major difference between anaerobic and aerobic breakdown relates to the transformation of NEtFOSE to NEtFOSAA. My next step was to create a potential biotransformation pathway that would display the differences in how NEtFOSE breaks down in anaerobic versus aerobic environments. I included the compounds that the lab results suggested were present within the pathway, as well as a few compounds that other studies suggest serve as intermediate metabolites. See Figure 4 (below, left) and Figure 5 (below, right).
Ultimately, the same metabolites were generated under both anaerobic and aerobic conditions, yet the concentrations of such compounds varied in some cases. The most significant differences in concentrations between the aerobic and anaerobic environments were the amount of NEtFOSE remaining and the amount of NEtFOSAA generated.
Conclusion
There are many gaps in the overall scientific understanding of PFAS. The EPA openly states on its website that its scientists do not fully understand how to efficiently detect and measure PFAS, how frequently people are exposed to PFAS, how harmful PFAS are to the environment, how to remove PFAS from water sources, and how to manage and dispose of the compounds (Environmental Protection Agency, 2021). This research project aimed to showcase how NEtFOSE degrades and transforms in aerobic and anaerobic environments, ultimately allowing us to better understand the fate and transport of the compound. Once scientists understand the types of compounds formed when NEtFOSE degrades, as well as the environmental conditions that support the formation of new compounds, they can best figure out how to remove and dispose of the compounds. Elimination of PFAS from natural and built environments will ensure the health and longevity of our ecosystems and of humankind.
Through my involvement with the Hamel Center for Undergraduate Research, I have fallen in love with research. I hope to stay involved in Dr. Mouser’s research group throughout my time at UNH. My REAP and SURF projects have prepared me for a career in environmental engineering, specifically related to water quality and pollution remediation. It is a dream of mine to work at the EPA as an environmental researcher after attending graduate school. The research process may be challenging, but in the end it is very rewarding.
I would not have been able to complete this project without the help of Dr. Mouser, Kellen Sawyer, and Lindsay Guertin. Thank you for your endless support! Thank you also to the Hamel Center for Undergraduate Research for funding my project, in particular Mr. Dana Hamel and Mr. John Greene.
References
Environmental Protection Agency. (2021). PFAS Explained. EPA. Retrieved January 12, 2022, from https://www.epa.gov/pfas/pfas-explained.
Rhoads, K. R., Janssen E. M.-L., Luthy, R. G., & Criddle, C. S. (2008). Aerobic Biotransformation and fate of N-ethyl perfluorooctane sulfonamidoethanol (N-EtFOSE) in activated sludge. Environmental Science & Technology, 42, 2873–2878.
Singh, R. K., Fernando, S., Baygi, S. F., Multari, N., Thagard, S. M., & Holsen, T. (2019, February 15). Breakdown Products from Perfluorinated Alkyl Substances (PFAS) Degradation in a Plasma-Based Water Treatment Process. ACS Publications. Retrieved July 29, 2022, from https://pubs.acs.org/doi/10.1021/acs.est.8b07031
What are PFASs Chemicals, and where are they found? Environmental Working Group – Know your choices. (2018). Retrieved January 12, 2022, from https://www.ewg.org/pfaschemicals/what-are-forever-chemicals.html.
Zhang, W., Pang, S., Lin, Z. Mishra, S., & Bhatt, P. (2020). Biotransformation of perfluoroalkyl acid precursors from various environmental systems: advances and perspectives. Environmental Pollution, 0269-7491.
Author and Mentor Bios
Alexis Eaton will graduate in spring 2024 with a bachelor of science degree in environmental engineering and a bachelor of arts in sustainability. Originally from Wallingford, Connecticut, she is a member of the University Honors Program, UNH's Society of Women Engineers, and Tau Beta Pi, UNH's engineering honors society. She also serves as an Honors Sustainability Fellow. Alexis’s participation in the Research Experience and Apprenticeship Program (REAP) in the summer after her first year at UNH sparked a love of research, leading Alexis to continue studying emerging contaminants with the support of a Summer Undergraduate Research Fellowship (SURF) in 2022. During this research project, she learned about the fate and transport of per- and polyfluoroalkyl substances (PFAS) in the water supply, and got to construct semi-permeable membrane bioreactors. Alexis plans to complete a PhD that continues her research focus on emerging contaminants and to pursue a career in the Environmental Protection Agency. As a second-time contributor to Inquiry, Alexis finds the journal to be a useful outlet to refine her communication skills with a general audience.
Dr. Paula Mouser, P.E., is a professor in the Department of Civil and Environmental Engineering at the University of New Hampshire. She joined UNH in August 2017; her teaching specializations are environmental engineering microbiology and environmental pollution, and her research focuses on microbial transformation of environmental pollutants. Mouser conducts research related to emerging contaminants, such as PFAS and PPCPs, that is funded by the New Hampshire Sea Grant. Alexis Eaton’s project dovetailed with research on this broader topic. Dr. Mouser enjoyed working with Alexis, who is one of the most motivated, independent undergraduate researchers she has mentored. She is pleased that Alexis took advantage of opportunities to work in the lab and the field and to learn from graduate students. Dr. Mouser feels that the process of writing for Inquiry is similar to writing a paper for a peer-reviewed journal: It requires substantial time to develop, multiple iterations of writing and editing, and patience to polish the paragraphs in a way that improves readability. In her words, “It’s an awesome experience for a student to be mentored through the technical writing process, and to have an opportunity to see their work in print for a wide audience to read.”
Copyright 2023, Alexis Eaton