Understanding the Behavioral and Neurobiological Mechanisms of Relapse in Alcohol Use Disorder
Alcohol use disorder (AUD), otherwise known as alcoholism or alcohol addiction, is a chronic brain condition that affects millions of people worldwide. While there are treatments available, there are still several challenges that lie in the way of successful recovery for those who are affected. One of the main challenges is people’s tendency to relapse during treatment.
Understanding how the brain functions with AUD, and specifically with relapse, is crucial to improving the treatment options for AUD. Further research into the neural and behavioral mechanisms behind relapse needs to be conducted before new treatments can be designed. Preclinical trials, such as this one, are using new research methods to bridge the gap between human and animal models to provide the necessary foundation for successful treatment research.

Hannah Manning
I became involved in Sergios (Sergey) Charntikov’s Lab at the University of New Hampshire in fall 2020, after deciding to pursue an interest in behavioral neuroscience. Dr. Charntikov, my mentor and an associate professor of psychology at UNH, studies the behavioral and neurobiological components of drug addiction in his lab using rodent models. I funded this research project with an Undergraduate Research Award (URA) in spring 2022 in partnership with Ethan O’Keefe; a Summer Undergraduate Research Fellowship (SURF) in summer 2022; and a second URA in spring 2023 in partnership with Kelsey Alimandi. Additional funding was provided by Dr. Charntikov through grants for the lab.
The goal of this study was to pioneer a new way of using preclinical rodent models to examine the neural mechanisms underlying relapse into alcohol addiction. We conducted a series of behavioral tests to determine the severity of the rats’ addiction-like tendencies. We predicted that rats with high demand for ethanol, or a higher addiction-like tendency towards ethanol, would show persistent responses in the face of negative consequences and show anxiety-like symptoms in the absence of ethanol (i.e., withdrawal). We also predicted that rats with high demand for ethanol would show a distinct neural profile compared to those with lower demand for ethanol. This study assessed this data at both a group and individual level to determine a vulnerability to relapse brain phenotype.
Background
Excessive alcohol use, including binge drinking and heavy drinking, is becoming an increasingly dangerous problem in American society. An estimated 95,000 people in the United States. die each year from alcohol-related causes, making it one of the leading preventable causes of death (NIAAA, 2022). In 2019, 14.5 million people, ages 12 and over, were reported to be diagnosed with AUD. (NIAAA, 2022). AUD is a chronic, relapsing brain condition characterized by cravings for alcohol, loss of control over drinking, and withdrawal symptoms without alcohol (Medline Plus, 2019). People who suffer from AUD continue to use alcohol in the face of negative consequences, such as legal, health, social, and economic problems (Yale Medicine, 2022). AUD can cause several physical health effects as well, including increased risk of injury, liver and heart disease, several different cancers, and poor pregnancy outcomes (CDC, 2022). There are several factors that can put someone at risk for AUD, including history of childhood abuse, family history of AUD, mood disorders, or other mental health conditions such as post traumatic stress disorder (PTSD) (Yale Medicine, 2022). See Figure 1.
There is treatment available for people who have been diagnosed with AUD. Treatment usually begins with medically managed detoxification, then maintained with therapies such as medication, counseling, and community support (Yale Medicine, 2022). Abstinence medications can be prescribed, while other supports come from cognitive behavioral therapy, family therapy, and mutual help groups such as Alcoholics Anonymous (AA) meetings (Yale Medicine, 2022).

Figure 1. Comparative visualization of alcohol consumption data between the United States and the rest of the world. Data sources include the CDC and NIAAA, as cited in references 1 and 2. Click to enlarge.
However, despite this, many people with AUD are not seeking help, or are not able to get it, with less than 10 percent of people with AUD in the past year receiving treatment (NIAAA, 2022). Even those who have access to treatment often find it extremely difficult to obtain or ineffective. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), 90 percent of alcoholics experience at least one relapse during their treatment (NIAAA, 2022).
There are several major factors implicated as the causes of relapse with AUD. Two prominent factors leading to relapse include stress and conditioned cues previously associated with ethanol (Lui and Weiss, 2003). Research has shown that stressful situations are associated with tendencies to relapse, and that certain environmental stimuli can become linked to drug use through Pavlovian conditioning (alcohol predictive cues), which can induce a relapse if those cues are presented during abstinence (Lui and Weiss, 2003). Therefore, there is a need to better understand the role of these factors in the relapse to ethanol use. Although there are studies investigating these effects on a group level, our study was designed to assess the role of these factors in relapse on the individual level.
Although AUD involves a significant neurobiological component, our understanding of it remains incomplete. The tendency for alcoholics to relapse indicates that alcohol addiction causes permanent changes in the brain (Aguilar et al., 2009). The fact that alcohol predictive cues remain salient, even after periods of abstinence from alcohol, implies that there are neural mechanisms underlying vulnerability to relapse (Jupp et al., 2011). There are numerous brain regions implicated in AUD relapse, and several neurotransmitter systems are involved (Aguilar et al., 2009). Abstinence from alcohol results in several neural alterations that may contribute to vulnerability to relapse. Despite what has been discovered so far, further research must be done into the neural mechanisms underlying vulnerability to relapse into alcohol use.
Behavioral Economics
Behavioral economics is an intersection between psychology, economics, and pharmacology, and its purpose is to characterize systematic decision-making mechanisms to help psychologists and economists understand why people make the decisions they do (Strickland and Lacy, 2020). A major contribution of the field is the concept of behavioral economic demand, which uses quantitative analyses to understand the consumption of a commodity at a given cost (Strickland and Lacy, 2020). For example, when studying drug addiction in humans, an economic demand model would examine how much money people are willing to spend to buy a given drug. Individual people will have different levels of demand for certain drugs, and some will be willing to spend more money than others, demonstrating a higher demand.
When implemented in a neuroscience laboratory setting, behavioral economics can create mathematical models for evaluating drug demand in animal trials (Strickland and Lacy, 2020). For example, an experiment assesses how many times a rat is willing to press a lever to receive an infusion of a given drug; the numbers of lever presses can be recorded and used to quantify the rat’s economic demand for that drug. The measure of lever presses is an indicator of how much the rat is willing to work, or “spend,” to have access to the drug, similar to how humans are willing to spend more or less money to have access to a drug. Behavioral economics allows for the quantitative alignment of human and animal research when it comes to drug addiction, and prior research has shown that economic demand models are strongly associated with addiction-like behavior in both human and rat studies (Bentzley et al., 2014). Principles of behavioral economics were used to structure this study’s experimental design.
Methods

Figure 2. Timeline illustration of the project, highlighting the different phases, responding in the face of negative consequences testing, and sucrose fading stages. Click to enlarge.
For this study, I was one of the undergraduate project leaders, in partnership with Ethan O’Keefe and Kelsey Alimandi. We worked with our graduate student leader, Anna Kalinowski, to coordinate the team of undergraduate research assistants and to make sure each phase of the project ran smoothly. Our project timeline and key experimental equipment may be seen in Figures 2 and 3.
We used 12 Wistar rats, six male and six female. The rats were housed in a climate-controlled vivarium, on a twelve-hour light-dark cycle. They acclimated to the vivarium and the research assistants for one week before the study began. All protocols involving the rats in this study were approved by the Institutional Animal Care and Use Committee (IACUC).
The rats self-administered ethanol in Med Associates operant chambers (30.5 x 24.1 x 21.0 cm (l × w × h)) for ten hours at a time, from 8 a.m. to 6 p.m. They administered the ethanol through nose poking. There are two small, recessed areas on the wall of the operant chamber, where the rat can insert its nose. When a rat pokes its nose into the hole in the wall of the chamber, it is presented with a drink of ethanol from a sipper bottle that extends into the chamber. This is an automated process run by our computer programs, which is software designed through MedPC. The rats

Figure 3. Essential project equipment: A visual showcase of the study's primary tools for self-administration and behavioral assessment, courtesy of Med Associates Inc. Click to enlarge.
administered on a variable ratio of 3 (VR3) schedule, meaning it would take between one to three nose pokes to receive a presentation of ethanol. The presentations of ethanol were accompanied by environmental cues from the chamber, including lights and sounds, which happen automatically when the bottle is extended. The repeated display of these cues during the presentations of ethanol created conditioned associations between the ethanol and environmental stimuli, and they would eventually function as ethanol predictive cues for the reinstatement portion of the study.
We introduced the rats to ethanol through a sucrose fading procedure. Rats often do not like the taste of ethanol, and it needed to be introduced with sucrose (which is a sweet tasting liquid) to ease them into drinking it. The rats began by drinking 12 percent sucrose solution from the sipper bottles in the operant chambers. Ethanol was introduced at two percent, then increased to four percent, eight percent, and 12 percent. Sucrose then faded out once ethanol concentration reached 12 percent. Sucrose solution concentration was reduced from 12 percent to eight percent, then to four percent, two percent, and zero percent, until the rats were just drinking 12 percent ethanol. This process took approximately three weeks. We then began the behavioral testing phase of our research.
Behavioral Testing
The first behavioral test we conducted was economic demand testing. This test determines how much the rats are willing to work in order to drink ethanol. Each rat participated in the test over two weeks, each being in the operant chambers every day for ten hours. The rats were placed in the operant chambers to self-administer, but each day it would take them more nose pokes to receive a presentation of ethanol. We achieved this through updating the operating program each day with the correct fixed ratio of administration. The rats would continue to nose poke until they failed to retrieve one presentation. Through prior literature research, we predicted that rats with low economic demand may not be willing to work more than 20-30 nose pokes for ethanol, while rats with high economic demand may be willing to work over 300-400 nose pokes for ethanol.
In this experiment, a single nose poke is a measure of cost: rats that are willing to “spend more” (administer more nose pokes) demonstrate that they have higher demand for ethanol than those that are willing to “spend less” (administer fewer nose pokes). By counting the number of nose pokes each rat administers, we were able to quantify and statistically analyze their demand.
The second behavioral test we conducted was negative consequence testing. This test measured how much the rats were willing to endure negative consequences to retrieve an ethanol presentation. During negative consequence testing, the self-administration session was split into a two-hour section and an eight-hour section. During the first two hours of the session, the rats would receive a mild foot shock through the floor of the operant chamber on 50 percent of their ethanol presentations, which was an automated process conducted through the operating software. We used eight different increasing levels of electric shock over the sessions (sessions including two hours of testing, eight hours of self-administration), with one rest day in between each of the eight test days (one level of electric shock per test day). The foot shock did not harm the rat but acted as a mild deterrent to drinking ethanol.
The third behavioral test we conducted was the elevated plus maze (EPM), the purpose of which is to measure the equivalent of withdrawal symptoms in rats. The EPM is an elevated cross which has two arms enclosed by walls and two arms open to the ground. The rats were placed in the EPM, and their movements were tracked by a camera placed above the maze. We observed the rats in real time during the session, and the footage was recorded and statistically analyzed after the test. We compared the percentage of time spent in the open arms of the maze to the percentage of time spent in the closed part of the maze. Based on prior literature research, we predicted that rats who were showing anxiety-like symptoms would spend more time in the closed arms of the maze, while rats who were not showing anxiety-like symptoms would explore the open arms of the maze. The tests took place one hour before the rats were supposed to begin self-administering ethanol each day over three days.
Extinction and Reinstatement
The final phase of the study was extinction and reinstatement. The purpose of extinction is to extinguish ethanol-seeking behavior and create a long period of abstinence without ethanol. The reinstatement is meant to mimic a relapse event in a human.
During extinction, we placed the rats in the operant chambers and allowed them to nose poke freely; however, there were no presentations of ethanol. After the two weeks of extinction, we placed the rats in the operant chambers for half an hour and presented them with the ethanol predictive cues (the light and sound cues presented during the bottle extension) they had during ethanol self-administration. The operant chamber tracked if the rats began to nose poke again in an attempt to retrieve an ethanol presentation using sensors in the chamber that automatically recorded movement to the operating program. This would show us if their ethanol-seeking behavior had been reinstated, or they were exhibiting relapse-like behavior.
One hour after the rats experienced reinstatement, they were euthanized with sodium pentobarbital and perfused using 0.9 percent saline and four percent paraformaldehyde. Perfusion removes blood from the brain and infuses paraformaldehyde to preserve the tissue for analysis. We then extracted the rat brains, which were processed in four percent paraformaldehyde and sucrose solution. The brains are currently being stored in a -80℃ freezer so that they can be examined later.
Current Progress on Analysis of Behavioral Tests
We have conducted preliminary analysis of the behavioral tests and the data is giving promising results. There is differentiation between rats with high economic demand and low economic demand for ethanol. The data is pointing to the fact that rats with high economic demand respond more in the face of negative consequences and have a higher propensity to relapse than those with low economic demand. In other words, the rats would be more likely to ignore negative consequences and continue to pursue ethanol due to higher addiction-like tendencies.
Grouped data is displayed in Figures 4, 5, and 6. While the existing data is supporting our predictions so far, more subjects need to be added to the project to reduce variation in data so that stronger conclusions can be drawn. There have already been two iterations of this study, one in the spring and summer of 2022 and one in the summer and fall of 2022. A third replication of this project is being conducted in spring 2023 with twelve more rats, as to double the subject number.
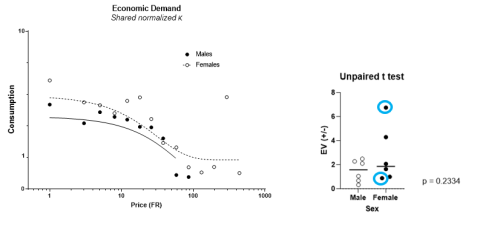
Figure 4. Economic demand test data: A graphical representation of the inverse relationship
between ethanol consumption (g ethanol/kg body weight) and price escalation (
schedule of reinforcement; left panel), highlighting potential individual differences (right panel).
Click to enlarge.

Figure 5. This graph plots the data from the responding in the face of negative consequences test,
showing a negative relationship between rewards earned (number of ethanol presentations retrieved)
and mild shock value (amps). The average responses for males and females are shown.
Click to enlarge.
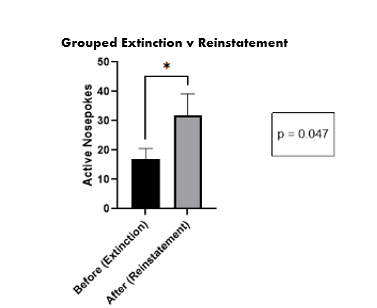
Figure 6. The average number of nose pokes during the extinction phase vs. the reinstatement.
Click to enlarge.
Next Steps
To evaluate the neural profiles of the rats, the next steps in the study will include processing the brains for imaging. The brains will be frozen, sectioned, and then will undergo c-Fos antibody staining. The processing will visualize a protein called c-Fos, which is an indicator of neural activity at the time of simulated relapse on the final test day. If a brain region was active during the reinstatement (i.e., relapse), that region will be identified with c-Fos immunohistochemistry. We will examine the processed brain sections with a confocal microscope to quantify the extent of c-Fos expression in different regions of the brain. Several brain regions will be examined while collecting neurobiological data, including the medial prefrontal cortex, orbitofrontal cortex, basolateral amygdala, central amygdala, the nucleus accumbens core and shell, and several more. Since these brain structures have all been implicated as regions involved in alcohol use relapse, we expect to see c-Fos activity in most, if not all, of these areas in rats who reinstated ethanol-seeking behavior. The neurobiological data will be processed in the summer 2023. Once the brain regions that are involved in vulnerability to relapse are identified, analyzed, and documented, we plan on attempting to inhibit some or all these regions to test the effects that it would have on the reinstatement, or relapse-like, event. If this project goes ahead, it will happen next academic year.
Conclusion
The experimental design of this study is a novel approach to understanding vulnerability to alcohol-use relapse. Previous research using rat models has consisted of short studies that only analyze the data as a group. However, in this study, we used a long-access self-administration model. Rats were allowed to self-administer ethanol for ten hours at a time and for several months, which is more like the way a human with AUD would consume alcohol. We are also examining the data collected at an individual level. Humans do not all consume alcohol in the same way, and not all people suffer from AUD; even those who suffer from AUD will not display symptoms and relapse in the same way. A similar phenomenon is observed in rats: not all rats will display the same addiction-like behavior when consuming ethanol. It does not provide an accurate analysis to only evaluate the data from rats as a group, as we would not do the same in humans.
While there are treatments available, including medication and therapies, there are still significant challenges faced by people recovering from AUD. The next step in improving treatment is to better understand neurobiology associated with relapse on the individual level. However, this is not yet possible as neuroscientists are still learning the neural pathways and brain regions involved in vulnerability to alcohol-use relapse. This study, while not directly treatment research, is a preclinical trial that is contributing knowledge of what makes a brain vulnerable to relapse into AUD, with the hopes of aiding clinical treatment research in the future.
I have gained many skills and learned so much during this research experience. I improved my leadership and teamwork skills, and practiced the responsibility that comes with leading a large research project. One of the most important things I learned was how to face challenges in research. This project came with many setbacks and difficulties, and it was up to the project leaders to overcome them and keep the project moving. I have become a more resilient researcher and gained a greater understanding of the overall research process. I am applying to graduate schools and feel that my undergraduate research has prepared me to move forward into a Ph.D. program. I plan to continue a career in research and am grateful for my experience thus far.
I would like to thank the principal investigator for this project, Dr. Charntikov, as well as the graduate student who oversaw the project, Anna Kalinowski. Thank you to my co-project leaders, Ethan O’Keefe, and Kelsey Alimandi, and to our undergraduate research team, Haily Knapp, Blake Tower, Megan Deane, Jessica Mulligan, and Karina Babina. Thank you also to the Hamel Center for Undergraduate Research for funding my project, in particular Mr. Dana Hamel.
Bibliography
Aguilar, M.A., M. Rodriguez-Arias, and J. Minarro. 2009. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Research Reviews 59(2): 253-277.
Bentzley, B.S., T.C. Jhou, and G. Aston-Jones. 2014. Economic demand predicts addiction-like behavior and therapeutic efficiency of OxyContin® in the rat. PNAS 111(32): 11822-11827.
Centers for Disease Control and Prevention. 2022. Data on Excessive Drinking (November 6, 2022) Retrieved from https://www.cdc.gov/alcohol/data-stats.htm
Jupp, B., E. Krstew, G. Deszi, and A.J. Lawrence. 2011. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin1 receptors. British Journal of Pharmacology 162(4): 880-889.
Lui X. and F. Weiss. 2003. Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse. Psychopharmacology 168: 184-191.
Medline Plus. 2019. Alcohol Use Disorder (AUD) (November 6, 2022). Retrieved from https://medlineplus.gov/alcoholusedisorderaud.html.
National Institute on Alcohol Abuse and Alcoholism. 2022. Alcohol Facts and Statistics (November 6, 2022). Retrieved from https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol....
Strickland, J.C. and R.T. Lacy. 2020. Behavioral economic demand as a unifying language for addiction science: Promoting collaboration and integration of animal and human models. Experimental and Clinical Psychopharmacology 28(4): 404-416.
Yale Medicine. 2022. Alcohol Use Disorder (November 6, 2022). Retrieved from https://www.yalemedicine.org/conditions/alcohol-use-disorder.
Author and Mentor Bios
Originally from Groton, Massachusetts, Hannah Manning will graduate in spring 2023 with a bachelor of science degree in biomedical science: medical and veterinary sciences and minors in zoology and classics. Hannah is involved in the University Honors Program, Phi Sigma Biological Honors Society, and Alpha Epsilon Delta Health Professional Honors Society. Hannah started working with Dr. Charntikov in September of 2020 as a lab assistant on a nicotine project and quickly became more involved after receiving a Summer Undergraduate Research Fellowship (SURF) to work on the same project in 2021. Now working on an ethanol project, Hannah has taken on an undergraduate leadership role. While working on different iterations of this project Hannah received an Undergraduate Research Award (URA) in partnership with student Ethan O’Keefe in spring 2022, a second SURF in 2022, and a second URA in partnership with student Kelsey Alimandi for spring 2023. This experience honed Hannah’s skills of project design, data organization, scientific writing specifically for research articles and grants, and presentation of research. Additionally, they learned to adapt and solve problems after needing to adjust their research focus and methodology from heroin to alcohol due to COVID-19 restrictions in place at the project's initiation. All these skills will be helpful in Hannah’s future goals of obtaining a Ph.D. in neuroscience, conducting research, and ideally working as a professor at the university level. After dedicating so much time and energy to the project, Hannah wanted to share the findings in Inquiry because they address such an important topic that is relevant to societal problems today.
Sergios (Sergey) Charntikov, an associate professor in the psychology department at UNH since 2015, specializes in behavioral neuroscience and substance abuse. Dr. Charntikov’s research focuses on understanding the long- and short-term effects of drug abuse, the underlying neuronal mechanisms associated with drug abuse, and relapse prevention techniques. Additionally, he studies how individual differences impact these contributing factors to drug abuse. Hannah's project is one part of a larger study exploring individual differences in substance abuse and serves as preliminary data. Dr. Charntikov was honored to work with Hannah, who he describes as one of the best students he has ever had. He finds her highly motivated, hardworking, and on her way to becoming a great scientist after graduate school. Dr. Charntikov acknowledges that Hannah's contributions to the project were invaluable and without her dedication and hard work, the project would not have been completed. Dr. Charntikov believes that clear and concise communication of scientific research to non-expert audiences is a vital skill for future scientists. Effective communication skills enable scientists to explain complex topics and findings to broader audiences and stakeholders in society, fostering critical thinking and inviting collaboration among peers. He is confident that Hannah's ability to communicate her findings will serve her well in graduate school and beyond, allowing her to continue contributing to the field of neuroscience.
Copyright 2023, Hannah Manning
